
Quality with ISO standards
Our ISO 13485 and certification supports our growth as a contact manufacturer of medical devices and components.
We have certified our Quality Management system for the contract manufacture of medical devices and their components in accordance with ISO 13485 standards. The standards focus on improving our risk management capabilities as well as developing our company operations. The certification process was handled by Kiwa Inspecta Sertifiointi Oy.
Screentec Oy is increasingly involved in the process of productization and manufacture of medical devices and their components for our customers. Our goal is to increase our market share in the segment of customers that require their suppliers to operate in accordance with the ISO 13485 standards. Screentec Oy strives to make the contract manufacture of medical devices and their components it’s largest product category, both in terms of turnover and profit.
In December 2019, Screentec has received its ISO 9001 certificate as well, further expanding our quality management systems and allowing us to provide ever better service to our clients.
You can download our ISO 13485 Quality Certificate HERE.
You can download our ISO 9001 Quality Certificate HERE.
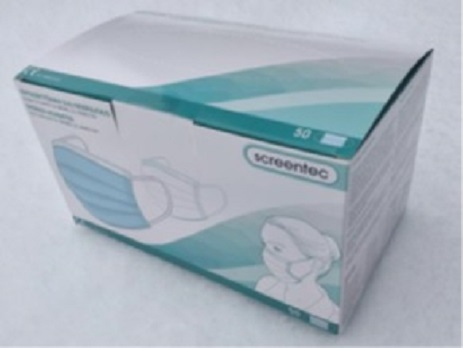
EU Declaration of conformity for Medical Face Masks
You can download EU Declaration of conformity for Screentec Medical Face Mask (type II, non sterile), Scr MFM02_CE, 009051797 from this link
EU Declaration of Conformity Scr MFM02_CE 009051797
You can download EU Declaration of conformity for Screentec Medical Face Mask (type IIR, non sterile), Scr MFM02_CE-IIR, 009052041 from this link

EU Declaration of conformity for Medical Visor
You can download EU Declaration of conformity for Screentec’s Disposable Visor Pro Elite 2 / 2S from this link
EU Declaration of Conformity Disposable Visor Pro Elite 2 009052675 / 2S 009052676
User Guide and Technical Datasheet for Screentec Medical Visor
Here is the user guide and technical datasheet for our medical visor: User Guide and Technical Datasheet for Screentec Medical Visor